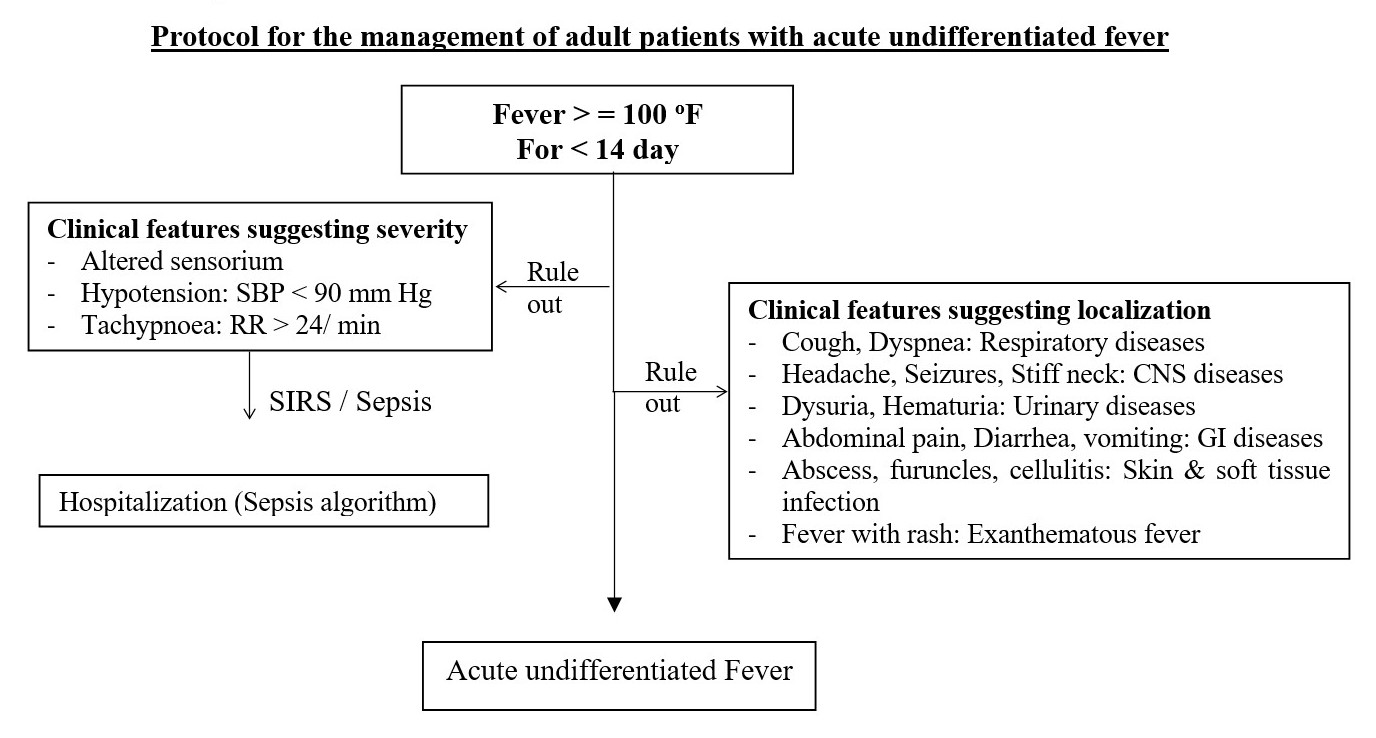
- No antibiotics are required for the majority of patients with acute febrile illness without an obvious clinical diagnosis.
- Start antibiotics for a presumed bacterial infection promptly, but adjust the drug's dosage and duration, switch to a new drug, or end antibiotic therapy when results do not support or justify the need to continue.
- Reassess the situation within 48 hours based on test results and patient status.
- Empirical treatment with doxycycline for patients with undifferentiated fever and negative rapid diagnostic tests for malaria and dengue is an option for the clinician.
- Day 1 or 2: Defer investigation and anti-microbials
- Day 3 or 4: Total leukocyte count with differential, Malaria parasite slide and rapid diagnostic kits, may test for Dengue if suspicion high.
- > 5 days: As per (B) plus paired blood cultures. May test for Dengue, Chikungunya, Scrub typhus, Leptospirosis if suspicion high.
- > 7 days: As per (C) plus X-ray chest and USG abdomen
- The first trimester of pregnancy- Treat pregnant women with uncomplicated P. falciparum malaria during the first trimester with either ACTs (there is increasing evidence of safety in this population) or the conventional regimen of 7 days of quinine + clindamycin.
- Infants less than 5 kg body weight- Treat infants weighing < 5 kg with uncomplicated P. falciparum malaria with ACT at the same mg/kg body weight target dose as for children weighing 5 kg.
- Patients co-infected with HIV- In people who have HIV/AIDS and uncomplicated P. falciparum malaria, avoid artesunate + SP if they are also receiving co-trimoxazole, and avoid artesunate + amodiaquine if they are also receiving efavirenz or zidovudine.
- Non-immune travelers- Treat travelers with uncomplicated P. falciparum malaria returning to non endemic settings with an ACT.
- Uncomplicated hyperparasitaemia (People with P. falciparum hyperparasitaemia are at increased risk of treatment failure, severe malaria and death so should be closely monitored, in addition to receiving ACT.
Preventing relapse in P. vivax or P. ovale malaria-
The G6PD status of patients should be used to guide administration of primaquine for preventing relapse. To prevent relapse, treat P. vivax or P. ovale malaria in children and adults (except pregnant women, infants aged < 6 months, women breastfeeding infants aged < 6 months, women breastfeeding older infants unless they are known no to be G6PD deficient, and people with G6PD deficiency) with a 14-day course (0.25-0.5 mg/kg bw daily) of primaquine in all transmission settings.
In people with G6PD deficiency, consider preventing relapse by giving primaquine base at 0.75 mg/kg bw once a week for 8 weeks, with close medical supervision for potential primaquine-induced adverse haematological effects. When the G6PD status is unknown and G6PD testing is not available, a decision to prescribe primaquine must be based on an assessment of the risks and benefits of adding primaquine. Pregnant and breast feeding women- consider weekly chemoprophylaxis with chloroquine until delivery and breastfeeding are completed, then, on the basis of G6PD status, treat with primaquine to prevent future relapse (Conditional recommendation, moderate-quality evidence).
If parenteral artesunate is not available, use artemether in preference to quinine for treating children and adults with severe malaria. Artemether is two to three times less active than its main metabolite dihydroartemisinin.
The initial dose of artemether is 3.2 mg/kg bw intramuscularly (to the anterior thigh). The maintenance dose is 1.6 mg/kg bw intramuscularly daily. This should be followed by a full dose of effective ACT orally.